Z7_89C21A40L06460A6P4572G3JN0
 Inglés UdeA - Cabezote - WCV(JSR 286)
Inglés UdeA - Cabezote - WCV(JSR 286)
Z7_NQ5E12C0L8BI6063J9FRJC1MV4
 Signpost
Signpost
Portal U de A
Z7_89C21A40L06460A6P4572G3JQ1
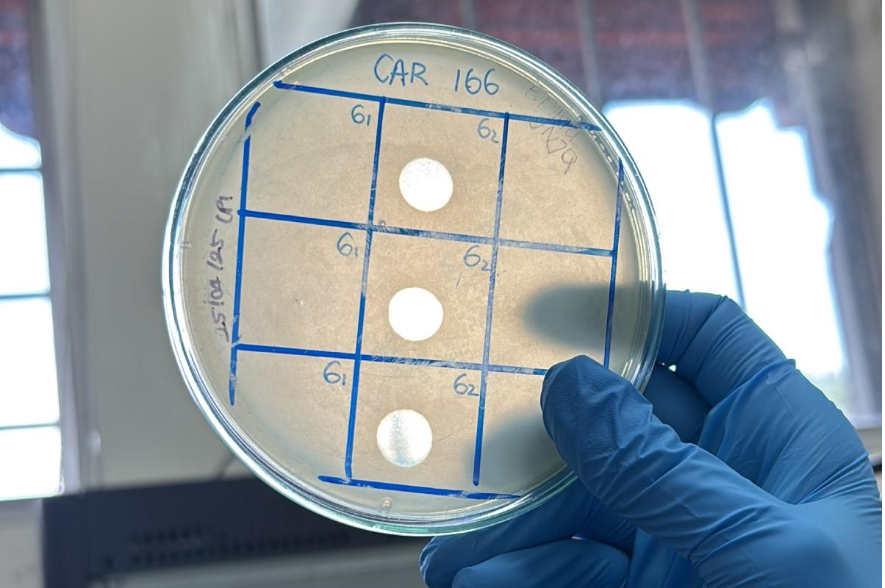
 They're here! Superbugs and phages wage a microscopic battle
They're here! Superbugs and phages wage a microscopic battle
Z7_89C21A40L06460A6P4572G3JQ3
 Portal U de A - Redes Sociales - WCV(JSR 286)
Portal U de A - Redes Sociales - WCV(JSR 286)
Z7_89C21A40L0SI60A65EKGKV1K57