Z7_89C21A40L06460A6P4572G3JN0
 Inglés UdeA - Cabezote - WCV(JSR 286)
Inglés UdeA - Cabezote - WCV(JSR 286)
Z7_89C21A40L06460A6P4572G3JQ2
 Signpost
Signpost
Generales
Z7_89C21A40L06460A6P4572G3JQ1
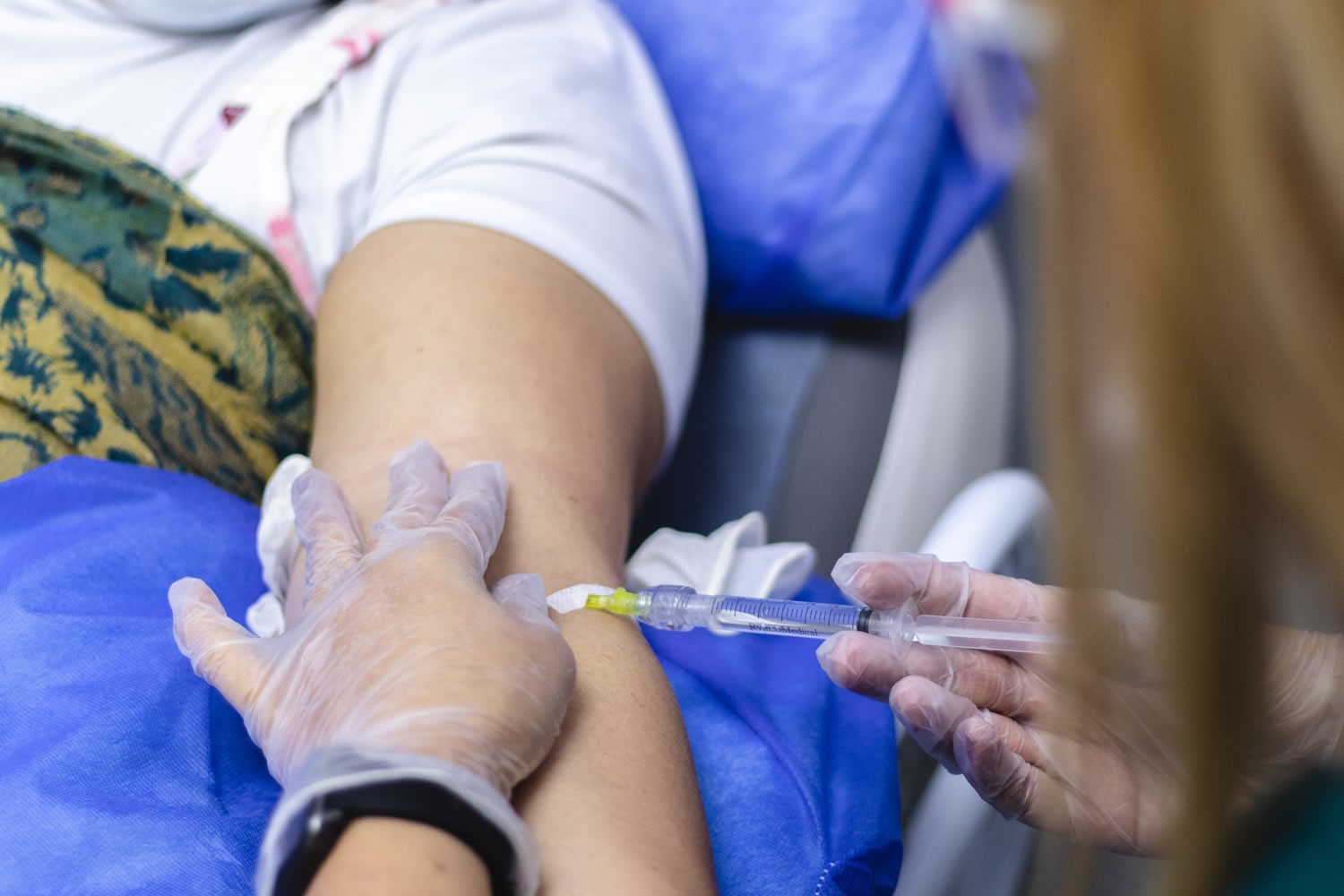
 Preliminary Results of UdeA's API Colombia Clinical Trial for the Prevention of Alzheimer's Disease Were Released
Preliminary Results of UdeA's API Colombia Clinical Trial for the Prevention of Alzheimer's Disease Were Released
Z7_89C21A40L06460A6P4572G3JQ3
 Portal U de A - Redes Sociales - WCV(JSR 286)
Portal U de A - Redes Sociales - WCV(JSR 286)
Z7_89C21A40L0SI60A65EKGKV1K57